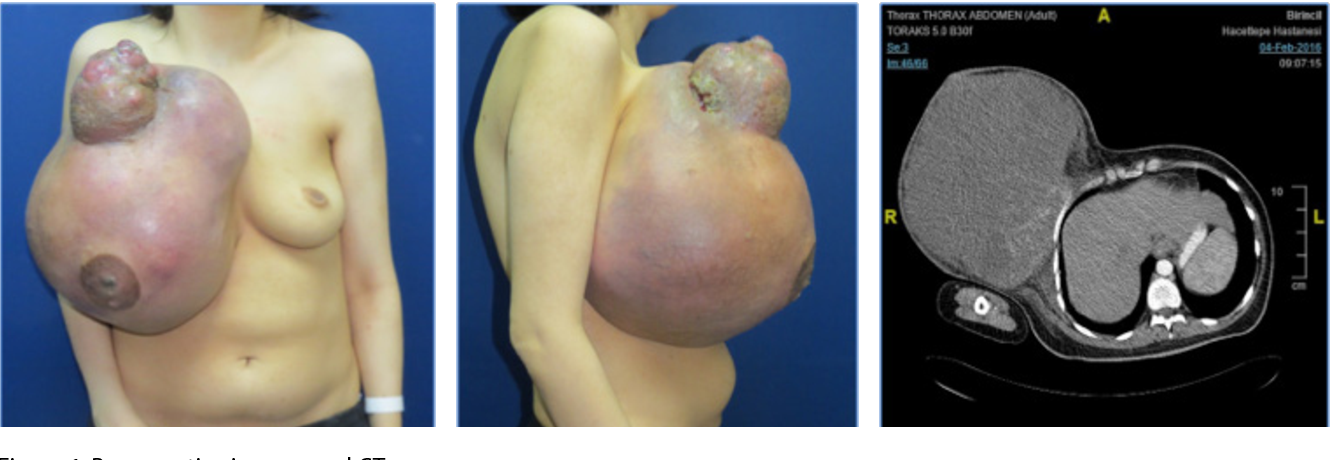
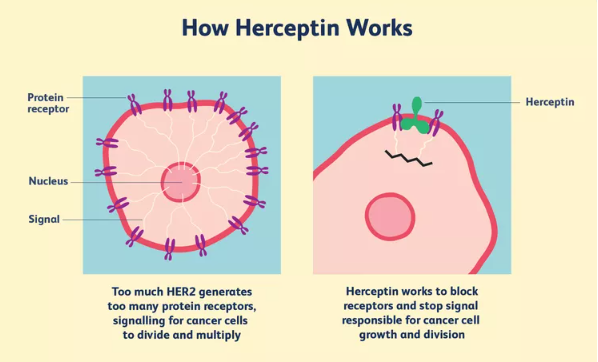
The FDA has approved a number of targeted therapies to treat HER2-positive breast cancer, including:
- Trastuzumab (Herceptin) has been approved to be used to prevent a relapse in patients with early-stage HER2-positive breast cancer.
- Pertuzumab (Perjeta) is used to treat metastatic HER2-positive breast cancer, and also both before surgery (neoadjuvant) and after surgery (adjuvant therapy).
- Trastuzumab and pertuzumab together can be used in combination with chemotherapy to prevent relapse in people with early-stage HER2-positive breast cancer. Both are also used together in metastatic disease, where they delay progression and improve overall survival.
- Trastuzumab deruxtecan (Enhertu) is approved for patients with advanced or metastatic HER2-positive breast cancer who have previously received a HER2-targeted treatment. A 2021 clinical trial showed that the drug lengthened the time that people with metastatic HER2-positive breast cancer lived without their cancer progressing. The trial also showed that it was better at shrinking tumors than another targeted drug, trastuzumab emtansine (Kadcyla).
- Tucatinib (Tukysa) is approved to be used in combination with trastuzumab and capecitabine (Xeloda) for HER2-positive breast cancer that cannot be removed with surgery or is metastatic. Tucatinib is able to cross the blood–brain barrier, which makes it especially useful for HER2-positive metastatic breast cancer, which tends to spread to the brain.
- Lapatinib (Tykerb) has been approved for treatment of some patients with HER2-positive advanced or metastatic breast cancer, together with capecitabine or letrozole.
- Neratinib Maleate (Nerlynx) can be used in patients with early-stage HER2-positive breast cancer and can also be used together with capecitabine (Xeloda) in some patients with advanced or metastatic disease.
- Ado-trastuzumab emtansine (Kadcyla) is approved to treat patients with metastatic HER2-positive breast cancer who have previously received trastuzumab and a taxane. It’s also used in some patients with early-stage HER2-positive breast cancer who have completed therapy before surgery (neoadjuvant) and have residual disease at the time of surgery.